There’s quite a lot of advances in solar we are not alone in the ball park
Let’s hope greater cell can pull this off
Solar Applications of Graphene
Download our Solar Applications of Graphene PDF
As our dependence upon renewable energy becomes more apparent, the need for efficient solar cells becomes more crucial, especially when they are one of the easiest and cheapest ways to generate clean energy. In general, solar cells are not that efficient. However, recent advances in graphene- based solar cells have seen the reflectance of solar rays reduced by 20%, which provides a potential efficiency increase up to 20%. There are currently many different variations of graphene-based solar cells being researched today. This guide gives a comprehensive overview into the different types that are being investigated by academic and corporate researchers around the world.
Principles of Graphene Solar Cells
The basic principle of a graphene-based solar cell is essentially not that different from current inorganic/silicon solar cells being produced today, with the exception that some of the materials currently in use are replaced with graphene derivatives. As with any device or material, there are parameters that can be improved to increase operational efficiency. Graphene excels in tune-ability and adaptability. For graphene-based solar cells, the two standout parameters that can potentially change the nature of the device are the number of graphene layers in the device (or in the individual components within a device) and the effects of doping a graphene-based material.
Effects of Graphene Layers in Solar Cells
The relationship between optical transparency, sheet resistance, and the number of layers can be characterized by a proportional decrease in both the optical transparency and the sheet resistance, with an increasing number of graphene layers. A single layer of graphene shows an optical transparency of 97.7%. A 3-layered graphene stack exhibits around 90.8% optical transparency and the addition of each layer corresponds to a 2.3% decrease in optical transparency. A single sheet of graphene produces a sheet resistance of 2.1 kΩsq-1 and 350 Ωsq-1, while retaining 90% optical transparency. The quenching effect of multiple graphene layers can be up to 11% greater than monolayer graphene, due to a higher hole accepting density.
Effects of Doped Graphene in Solar Cells
The doping of heteroatoms onto a sheet of graphene can significantly alter the chemical, physical, electronic and photonic properties of the sheet and is a common approach in many solar cells. There are two main types of doping- p-type and n-type. P-type doping utilizes trivalent atoms, such as boron, which extracts an electron off the graphene sheet and creates a hole, a process known as hole doping, where the hole is created in the valence band of the graphene sheet. Whereas, n-type doping involves pentavalent atoms, such as phosphorous, and is an electron donating doping approach that facilitates a free electron from the pentavalent atom onto the graphene sheet. The free electron in this instance is facilitated in the conductance band of the graphene sheet.
Doping a graphene sheet can occur through various methods, including through solid, liquid and gaseous phase chemical doping, ball milling, thermal annealing, in-situ doping during chemical vapor deposition (CVD) methods and plasma treatment, to name a few. The effect of doping varies depending on both the type of graphene derivative used and the doping process. Regardless of which of these parameters (or both) are utilized in the doping process, the general result is improved efficiency of the solar cell.
Graphene-Silicon Solar Cells
Various allotropes of carbon have been implemented into solar cells to reduce the cost, allowing them to be more widely used. Other allotropes of carbon, have not been successful due to the inability to tune the electronic properties and the thickness of the layers. Graphene based films for solar cells can be produced with a predetermined thickness and complete coverage. It also allows the properties to be tuned, dependent upon the doping mixture used. Graphene has now been implemented into various junctions in graphene-silicon solar cells, including p-type heterojunctions, n-type heterojunctions and Schottky junctions. Graphene-silicon solar cells are being researched however pure silicon cells performance is still superior. The tuneability of graphene is promising for hybrid solar cells. While it is not at the same level yet, advancements are being made and it is just a matter of time until their efficiency surpasses pure silicon cells. To date, n-type heterojunctions can generate a 0.55- 0.57 internal voltage to help facilitate electron-hole separation. Schottky junctions have only showed a power conversion efficiency (PCE) of 1.5%, but the fill factor at present has only reached 56%, so theoretically, the efficiency can be vastly improved upon. Doping the graphene layers with gold particles has found to increase the efficiency by up to 40%.
Graphene-Polymer Solar Cells
A highly researched area of graphene incorporation is in polymer-based solar cells. Polymeric materials offer many advantages over inorganic-based materials due to their tuneability, low-cost and simple fabrication processes.
Graphene has shown great potential in transparent electrodes as a replacement for indium tin oxide (ITO) in polymer-based solar cells. The graphene in the electrode becomes an organic-inorganic hybrid material after it undergoes coating, layering, reduction and temperature annealing. The hybrid material has a better energetic relationship, as the fermi-level of the graphene and the semi- conducting layer are closer together for an efficient charge injection. Graphene-polymer transparent electrodes also possess a high work function and conductivity, but it does have a limit of 65% light transmittance. In addition to reducing the graphene into hybrids, CVD-produced graphene can also be used as transparent electrodes. CVD-graphene is ozone treated, which produces carbonyl and hydroxyl functional groups on the surface of the graphene. The oxygen based functional groups improves the open circuit voltage, but conductivity is reduced due to the sp2 hybridized covalent network being disrupted by sp3 bonds around the functionalized carbons. Non-covalent functionalized CVD-grown graphene shows a good conductivity and can have up to 0.55 V open circuit voltage, a fill factor of 55% and a PCE of 1.71%. The flexibility of graphene allows the solar cell to bend up to 78° more than pure ITO electrodes.
Electron transporter and acceptor based graphene-polymer solar cells rely on a high electron affinity to dissociate the electron-hole pairs into separate charges. Unlike other materials, graphene gives and effective separation when mixed with conjugated polymers. The large surface area of graphene allows for a continuous pathway and multiple donor/acceptor sites for efficient electron transfer. This type of solar cell has produced a PCE of 1.1%.
A hole transport layer is required in many solar cells to stop current leaking and charge recombination. Graphene can be mixed with polymeric material to produce a material with a band gap of up to 3.6 V which prohibits electron migration from the cathode to the anode. A 2nm graphene film is known to provide the best results as the thick film prevents the transmittance of electrons and increases electrical resistance. The highest PCE obtained has been 9 %, which is comparable, if not greater, than other materials used as hole transport layers.
Dye Sensitized Solar Cells (DSSC’s)
DSSC’s are different when compared to other types of solar cells. They contain a semi-conducting material (e.g. TiO2) with a photo-sensitive dye as the anode coupled with a pure metal cathode (e.g. Platinum) and an electrolyte solution. Graphene has many favourable properties that can increase the loading efficiency of the dye molecules, increase the interfacial area and improve the conductivity of the electrons to compete against the effects of charge recombination. Balancing the ratio of TiO2 and graphene is crucial to achieving an efficient system. The valence electrons from graphene become excited into the TiO2 conduction band via the graphene-TiO2 interface, which efficiently separates the electrons and the holes. So, enough graphene is required (roughly 1%) to facilitate this separation, but the introduction of higher graphene concentrations into the matrix causes the transmittance to be reduced. The incorporation of graphene into DSSC’s improves the light scattering at the photoanode, efficiently disperses the dye molecules and provides an efficiency that is 39% greater than pure TiO2 electrodes.
Graphene/Quantum Dot (QD) Solar Cells
Both graphene and carbon nanotubes have been hybridized with quantum dots to make functioning solar cells. Of the two carbon allotropes, graphene hybridized quantum dots have shown the most potential. Produced by electrophoretic and chemical bath deposition on ITO, a layered structure of both graphene layers and CdS quantum dots can be produced. The optimal layering structure consist of eight repeating graphene-CdS bilayers. This graphene-CdS configuration can produce and efficiency of up to 16%, which out performs carbon nanotubes-CdS by 7%, and 11% for other carbon allotropes. This is attributed to graphene producing a better scaffold to incorporate the quantum dots, the layered structure provides a fast electron transfer from the QD to the graphene while suppressing the recombination of charges.
Graphene-Tandem Solar Cells
Tandem solar cells, otherwise known as multi-junction solar cells, are composed of two or more sub- cells that are stacked together in either a series or parallel configuration. It has been predicted that a single solar cell can theoretically produce up to 40% solar energy conversion efficiency, but tandem solar cells have the potential to reach up to 86% efficiency. The PCE of many solar cells has been enhanced to date by employing tandem arrangements.
The use of low band-polymer hybrid solar cells, commonly using ITO and other carbon derivatives, has been well studied, but graphene-based tandem solar cells are still a relatively new field. There has, however, been some promising developments using graphene oxide. Graphene tandem solar cells have not yet reached the heights of their non-graphene counterparts, but as a relatively new area they show great potential, especially as non-graphene tandem solar cells show relatively high PCEs.
One such development is that of a graphene oxide and polymer tandem solar cells that consist of 2 sub-cells. The cells consist of a bilayer of Cs-neutralized graphene oxide and pure graphene oxide connected by a charge recombinant layer of MoO3 and aluminium. Such cells have been found to produce a PCE between 2.92% and 3.91%, depending on the polymer blend used and the thickness of the different components within the cell. The open circuit voltage of the cell can vary between 1.23 V and 1.69 V, but is dependent on the resistance of the interconnecting layer between the graphene oxide sheets, which is a function of the thickness of the layer.
Another tandem cell that utilizes graphene also incorporates single-walled carbon nanotubes. Combinations of these materials have also been used as the hole transport and interconnecting layers for ITO-based sub-cells. The thin film composed of these two carbon allotropes have been used in both regular and inverted solar cells, and the associated solar cells exhibit PCEs of up to 3.50% and 2.90%, respectively. The resulting solar cell possess a higher PCE than solar cells that contain the same sub-cells but lack the graphene connecting layers. Even though in this application they are not directly involved in the sub-cells, the presence of graphene in the device increases its overall efficiency.
Graphene-Perovskite Solar Cells (PSCs)
Perovskite solar cells (PSCs) have made great strides over the last few years due to their interesting bandgap and absorption properties that produce high PCEs. Perovskite solar cells have a standard structure, including the type of materials that are used, so the substitution of one material for another is a relatively simple process that leads to highly tuneable solar cell devices.
Nanocomposites composed of anatase-TiO2 and graphene nanoflakes have shown promise with a PCE up to 15.6%. The best results in these solar cells can be achieved when the nanocomposite is utilized as an n-type electron collection layer. The graphene is present as a monolayer and is only present as 0.6 wt% of the whole cell. Any amount above this drops the efficiency and is dependent upon a thin collecting layer. These perovskite solar cells can also be produced by low temperature sintering methods. These cells also possess short circuit and open circuit values of 12-21.9 mAcm-2 and 1.05 V, respectively.
When paired with an efficient light absorber, graphene oxide can be used as a hole conductor for inverted solar cells. The fabrication of this class of PSC is more complex in its synthesis, but provides a PCE between 9.26% and 11%, which is up to 7% greater than similar solar cells without the graphene oxide layer. Thinner layers of graphene oxide (2nm) can produce higher efficiencies. The average short and open circuit values in these solar cells are around 15.58 mAcm-2 and 0.99 V.
Similar solar cells to the previous example have been created, but by using reduced graphene oxide as a hole transport layer, with a light absorber material. These solar cells only reach a PCE maximum of 9.14%, but are much more stable and can retain 62% of their initial PCE after 140 hours of constant sun exposure. Thus out performing many other solar cells that can deteriorate significantly after 120 hours. The higher stability is attributed to the increased resistance that reduced graphene oxide possess against oxygen and moisture compared to other graphene derivatives.
One application of graphene oxide is to functionalize it with amphiphilic moieties to promote interface wettability on the surface of a perovskite solar cell. The modification of graphene oxide can reduce the contact angle of hole transporting layer solutions to 0°. The carbon-carbon bonds in the graphene sheet absorb the hole transport layer molecules via p-p interactions and improve the both the interfacial interactions within the solar cell which leads to an improved performance. The functionalized graphene oxide can be doubled up as a buffer layer in PSCs and the dual-purpose graphene sheets can not only increase the short and open circuit potentials, but also increase the PCE of a PSC device by up to 45%.
The graphene derrivative graphyne can be used in PSCs to achieve high results. Graphyne is a 2D material similar to graphene, but unlike graphene’s structure of a regular hexagonal sp2 array, graphyne possess a mixture of sp and sp2 hybridised carbons and can be thought of as a lattice of phenyl rings connected by acetylene bonds, which arrange themselves as irregular hexagons. The incorporation of graphyne into hybrid electrodes, in an inverted solar cell, can achieve PCEs up to 14.8%- much higher than non-graphyne solar cells of a similar composition.
Graphene-Organic Solar Cells
While the main focus around solar cells generally tends to involve the different inorganic components, the organic components of the solar cell also play a major role. Organic and inorganic components in a solar cell have advantages and disadvantages, but the optimization of the organic components can produce a more efficient solar cell.
Components that might traditionally be inorganic in nature are now being replaced with inorganic- organic hybrid materials that offer greater physical properties, solution-processability, cost-effective production, a large surface area, and are much lighter in nature. One concern with many solar cells is the environmental stability, but organic molecules can provide stability against temperature, moisture and chemical degradation in solar cells, even when present as a hybrid material. The combination of organic and inorganic components generally produces higher stabilities and efficiencies than their pure predecessors.
Aside from the two electrodes, traditional organic solar cells contain an active PEDOTSS layer and a donor-acceptor blend layer- commonly composed of P3HT or fullerenes (or both). In recent years, the active PEDOT
SS layer has been replaced by graphene derivatives and are generally used as hole-transport layers in organic solar cells. These components, while not specifically a class of their own, cover a wide range of solar cell applications nowadays, including in many heterojunction solar cells.
Graphene Bulk-Heterojunction Solar Cells
Graphene’s high electronic conductivity, transparency and flexibility make them useful in heterojunction solar cells, where they can be employed in many different ways including electrodes (both anodes and cathodes), acceptor layers, donor layers, buffer layers and active layers. The multijunction within the solar cell relies heavily on graphene’s specific tuneable parameters, including the thickness, thermal annealing temperature, the concentration of doping on the sheet and its photovoltaic performance.
Graphene-heterojunction solar cells are by far the most widely studied and used graphene-based solar cell. There are many variations of heterojunction solar cells and how graphene derivatives can be incorporated into them, including as transparent electrode, photoactive layers and Gallium Arsenide (GaAs) solar cells. As such, graphene heterojunction solar cells cannot be generalized as a single class of solar cells.
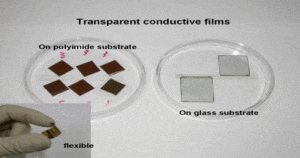
Graphene Transparent Electrodes
Graphene can easily be incorporated into certain layers. Coupled with its excellent electrical, optical, mechanical, and thermal properties, this has allowed graphene to be studied as transparent electrodes in solar cells. We’ve explored a few of the different molecules employed in composite graphene transparent electrodes, but there are many others currently being researched.
Prior to graphene being employed as a transparent electrode, ITO was the most commonly used material because of its high optical transparency. However, ITO is not cost effective, is brittle, and lacks mechanical flexibility. Graphene exhibits a high optical transparency 90-100% and a low sheet resistance, even in multiple layered graphene stacks- both of which are great properties for transparent electrode applications.
There have been many cases of graphene derivatives being employed as both the anode and the cathode in heterojunction solar cells. Some of the common molecules used in these graphene derivatives including polyethylene naphthalate (PEN), PEDOT, PSS, MoO3 and ZnO, to name a few. PEDOTSS layers are the most common in graphene transparent electrdoes, with other materials being incorporated to improve and/or tune the properties.
Using a graphene-based dual electrode system, flexible solar cells have been fabricated using different graphene derivations. PEN substrates containing various combinations of graphene, PEDOTSS, PTB and inorganic oxides generally show a PCE of 6.1-6.9% for the anode and 6.7- 7.1% for the cathode. These solar cells have also been found to exhibit a short-circuit photocurrent density up to 14.8 mAcm-2, an open circuit voltage up to 0.71 V, with the potential to obtain a form factor of up to 57.6% after 100 tensile flexing cycles (or 20 flexing cycles). Solar cells from these composite dual graphene electrodes have found to show no loss of activity under mechanical tension binding tests, a high efficiency and excellent mechanical strength.
Another range of graphene bi-electrode solar cells incorporating PEDOTSS, copper and Buckminster fullerenes (C60) into the electrodes, where one electrode is doped with gold particles (in the form of gold (III) chloride). The doping of the graphene electrode changes the wetting in the PEDOT
SS layer on the surface of the graphene sheet. The change in properties leads to an enhances PCE performance across the whole cell. Solar cells of this variety have been produced using 1-3 layers of graphene sheets. The solar cells electrodes can have a sheet resistance between 300 and 500 Ωm-2, with a transmittance ranging between 91.2-97.1%. The PCE of such solar cells are found to be around 1.63%. The PSS can be replaced by polyethylene glycol (PEG) to produce a solar cell which is less corrosive in nature (PSS is a strong acid). However, compared to other cells, the PCE is much lower so it’s not generally used.
An alternative to using PEDOTSS is MoO3. This has been used by some researchers to produce a different kind of hole transport layer. In these cells, low pressure CVD-grown graphene is used as the graphene source. These cells consist of anode composed of graphene, MoO3, C60 and copper phthalocyanine (CuPc). This is a similar composition to many PEDOT
SS electrodes and allows for a direct comparison in performance- one of the many reasons why the composition was chosen. Depending on the thickness of the hole transport layer, the PCE of these solar cells can range from 0.71-0.31%. While the PEDOT
SS reference cell exhibited a PCE of 0.85%, this cell class exhibit a much higher PCE than transition metal electrodes, where Mg/Al electrodes exhibit the highest at 0.56%.
Another area of graphene transparent electrodes research explores zinc-graphene anodes. Some researchers have developed a hybrid photoanode, based around a P3HT, ZnO and ZnS core-shell nanorod array, suspended on a reduced graphene oxide film modified with ITO. Each component has a specific function within the electrode, the P3HT acts the hole acceptor, the ZnS as the mediator and the ZnO is transporter and conductive collector. In these anodes, the PCE is enhanced by the presences of both a reduced graphene oxide film and ZnS/ZnO nanorods, showing a PCE greater than 1.01%. This is two and half times the PCE of the electrodes that don’t contain graphene, although still not as high as other solar cells.
There have also been many other attempts to improve the photovoltaic performance of both the transparent electrodes and the solar cells as a whole. Various multilayer electrodes based around graphene, gold, P3HT, PCBM, PEDOTSS, copper and PMMA in various compositions have been employed with significantly different results. The transparency of these electrodes varies from 82.3% to 90% with sheet resistances varying massively between 92 Ωm-2 and 374 Ωm-2. The PCE of such electrodes can vary between 1.17% and 13.3%.
Graphene Photoactive Layers
Both graphene and graphene oxide can also be employed in heterojunction solar cells as photoactive layers in the form of an active interfacial layer, electron-hole separation layer, hole-transport layer or as an electron-transport layer. As a general class of materials, graphene photoactive layers can exhibit a PCE from anywhere between 0.4 and 10.3% depending on the graphene derivative and the type of photoactive layer being produced. There are currently hundreds of graphene photoactive layers being employed as heterojunction solar cells.
Photoactive layers composed of a few layers of pure graphene films whether produced by CVD, flame pyrolysis, or other exhibit a PCE range between 1.01-2.88%. They can be employed as n-type heterojunctions by utilizing n-type type silicon alongside the graphene layers. Doping with nitric acid increases the PCE of these pure graphene heterojunctions up to 4.35%, where up to 4.18% of the PCE can be retained after 10 days. Graphene can also be coated onto n-type silicon nanowire arrays, where the nanowires suppress and harvest light much better than their planar counterparts. However, they do show a lower PCE value than planar graphene-silicon heterojunctions, even after doping with thionyl chloride. Planar graphene-silicon solar cells can also be doped with thionyl chloride show a PCE lower than nitric acid doping, but greater than that of pure graphene-silicon heterojunctions with a PCE of 3.93%. The highly volatile nature of thionyl chloride is responsible for the lower doping effects compared to nitric acid.
The interface of the heterojunctions is the most important part and a single layer of CVD-grown graphene (97% transparency, 350 Ωm-2) on silicon can exhibit a PCE of 5.38-7.85%- much greater than multilayer graphene heterojunctions. This value can be increased even further to 8.94% by the incorporation of an antireflection layer of silicon dioxide.
Aside from pure graphene, many graphene hybrid materials exist as photoactive layers. One such example is that of lithium neutralized graphene oxide (GO-Li) as an interfacial layer between the photoactive layer and electron transport layer of solar cells. The incorporation of such layers can increase the PCE of a solar cell by up 6.29% compared to solar cells without the GO-Li layers. The thickness of these layers can tune the photovoltaic performance, with thicker layers producing a higher increase in the PCE. This layer also improves the stability of the solar cell under solar exposure, moisture and air.
Graphene quantum dots and crystalline silicon can be used as electron blocking layers to prevent charge carrier recombination at solar cell anodes. In these cells, surface passivation can occur due to differing terminal groups, namely, oxide, hydrogen and methyl moieties. Cells containing the methyl terminal group show the best PCE of up to 6.63%, compared to 2.24 and 2.92 for hydrogen and oxide terminal groups. However, degradation can occur over time and the short circuit value can drop by more than 5 mAcm-2 and the PCE can drop by up 1.2%.
Graphene oxide can be used with gold nanoparticles to produce anodic buffer layers. Capping agents are utilized in these hybrids, generally in the form of glycine or sodium citrate and can show a PCE ranging from 2.82-3.34%. However, the inclusion of P3HT and IBCA into the solar cell can increase the PCE up to 5.10%.
Graphene oxide nanoribbons (GORs) can be used a hole extraction layers in many solar cells. These layers have been developed to replace existing ITO-based materials and have so far managed to increase the PCE of a solar device from 2.20% to 4.19%. Aside from the PCE, the incorporation of GORs produce a lower sheet resistance and a higher shunt resistance compared to their ITO counterparts.
Electron extraction materials in solar cell devices can be fabricated using Cs-neutralized graphene oxide. Solar cells utilizing these materials have been found to exhibit PCEs up to 3.67%. However, more importantly, the photoactive layer has been found to operate independently to the electrode materials, in both normal and inverted devices. The charge neutralization ability of these materials can reverse the charge extraction properties in heterojunction solar cells.
One of the most efficient graphene photoactive layers is produced from a hybrid material containing graphene oxide, PEDOTSS and n-type silicon nanowires. The wt% of graphene oxide has a profound effect on the PCE of the device with the optimum concentration being 30%, which produces a PCE of up to 9.57%. In comparison, the substitution of silicon nanowires for planar silicon produces a massive drop in the PCE to 4.30%. These layers not only show a high optical transparency compared to non-graphene photoactive layers of a similar composition, but also exhibit a reduction in the exciton decay.
Graphene Schottky Junction GaAS Solar Cells
GaAs solar cells have been one of the most widely studied type of heterojunction, namely Schottky junction, solar cells. Despite the large amount of research, only a few have reached PCE levels comparable to that of other heterojunctions and PSCs. However, the ones which have achieved high PCEs are some of the most efficient graphene-based solar cells. GaAs has a superior band-gap to silicon, with a charge carrier mobility that is six times higher. Theoretically, GaAs heterojunctions have the potential to produce efficient solar cells, but the devices currently being produced vary in quality.
One of the cells that is less favorable than it’s high-flying counterparts is based around CVD-grown single and multilayer graphene on n-type GaAs substrates, which only shows a PCE of 1.95% and an open circuit voltage of 0.65 V. Another such example is that of pillar-array-patterned silicon substrate with graphene, which only shows a PCE up to 1.96%. With nitric acid doping, the cell can achieve a PCE of up to 3.55%, but it’s still lower than many other solar cells. By comparison, a Schottky junction solar cell made from CdS nanowires and graphene has only achieved a PCE of 1.65%, showing that there is a wide range in terms of quality, not only with GaAs solar cells, but with Schottky junction solar cells in general.
Of the higher achievers, one example is that of a solar cell composed of an n-type silicon and TFSA- doped graphene Schottky junction. The synthesis approach is simple and a PCE of up to 8.6% can be achieved, which is 4.5 times higher than that of its un-doped counterpart and 6 times greater than other GaAs solar cells. The doping of TFSA on these devices not only increases their performance, but also enhances the stability of the device, compared to an un-doped version, to both oxygen and moisture.
One of the better-quality GaAs solar cells is that of a cell which is composed of a GaAs substrate and graphene, with a silicon nitride (SiNx) insulating layer and silver ‘fingers’. These solar cells have achieved much better efficiencies than many other solar cells, with PCEs varying between 10.4% and 15.5%. By optimizing the open circuit voltage, junction ideality factor, graphene resistance and the internal interfacial contact, there is a theoretical possibility to achieve a PCE of up to 25.8% with these solar cells.
Solar cells composed of graphene/semiconductor van der Waals Schottky diodes, with a tuneable gate and Fermi level, lead the way in terms of efficiency. The heterojunction utilizes a graphene- dielectric-graphene gate to achieve a PCE of up to 18.5%- much higher than other GaAs solar cells. The open circuit voltage, while not the best compared to other solar cell classes, is better than many other GaAs solar cells, with a value of 0.96 V. Aside from producing a highly efficient solar cell, there are theoretical predictions that the PCE of these cells could be increased to 23.8%.
The last two GaAs solar cells, show values close to commercially ready solar cells, and until recently, silicon-based solar cells had only reached a PCE of up to 22.5%. So, with a bit of optimization, and despite the discrepancies in quality over the whole class of solar cells, some GaAs could reach efficiencies comparable to commercial solar cells. Recent developments in silicon-based solar cells has achieved a PCE of up to 26%, but this has only just been discovered and is currently confined to academic laboratories.
Graphene Solar Cells Design of Experiments
Graphene Transparent Electrode
To produce a graphene transparent electrode for heterojunction solar cells, first, produce or purchase CVD-grown graphene on copper foil. To prepare this electrode, a modified transfer method is needed. To transfer, deposit PMMA and cure, followed by etching of the copper foil with FeCl3 solution and rinse with deionized water three times. The next stage is to rinse the PMMA-graphene with deionized water and place on a glass substrate. Re-deposit one drop of PMMA onto the material, cure and remove the PMMA with acetone. Dope the transferred graphene film with HNO3 vapor (69% concentration, 10 seconds).
To fabricate the device itself, the preparation of a silicon wafer with PEDOTSS is required. Clean a silicon wafer with acetone, ethanol and deionized water for half an hour and treat through chlorination and alkylation. Incorporate PEDOT
SS with DMSO (5 %wt) and Triton (1 %wt) and stir to ensure through mixing.
The fabricate the cell itself, use physical vapor deposition (PVD) and deposit LiF (0.6 nm) and Al (200 nm) electrodes onto the back of the silicon wafer. Spin-coat the PEDOTSS solution onto the silicon wafer and graphene-glass substrate (4000 rpm, 1 minute, 70-80 nm thickness). Anneal the organic films (125 °C, 30 minutes) in a glove box. Encapsulate the solar cell, using a clamp and AB glue to firmly stick the silicon wafer and the graphene-glass substrate together.
Graphene Ga/As Solar Cell
The promising Ga/As solar cell has the potential to be further optimized for commercial use.
Firstly, purchase or grow CVD-graphene on copper foil. Next, remove the oxides on the Ga/As wafers by dipping them in HCl solution (10 %wt, 3 minutes) and attach gold contacts (60 nm thickness) onto the back surface of the wafer by thermal evaporation. Deposit a SiNx layer (80 nm) on top of the Ga/As surface by plasma enhanced CVD with a lithography-processed mask, to act as the insulating layer between Ga/As and graphene. Open a window (active area) on the Ga/AS by dipping in HCl solution (10 %wt, 5 minutes) and rinse with deionized water. Treat the active area using NH3 plasma treatment (5 min with a 120 W 27.5 MHz RF generator).
Transfer the graphene sheet onto the substrate using PMMA as a support. Remove the PMMA with acetone and paste silver onto the graphene, above the SiNx area, followed by annealing (120 °C, 5 minutes). Spin coat TFSA (bis(trifluoromethanesulfonyl)amide) to dope the graphene. Add an antireflection layer- an electron beam evaporated Al2O3 film (68 nm thickness). To prepare the gate, transfer an extra layer of graphene onto the active area that is coated with Al2O3 by the same method as before. Remove the PMMA and paste the silver gate electrode onto the graphene gate, followed by annealing (120 °C, 5 minutes).
Graphene Solar Cells Future Advancements
Solar cells are a topic of intense research in academia and industry alike with new advancements being realized all the time. Most solar cells being produced utilize silicon and inorganic-based materials, which are at some point going to reach their limitation. The incorporation of organic molecules of graphene derivatives, low band-gap polymers, or both, are set to revolutionize the industry and lead to many commercially viable solar cell device architectures. There has been tremendous progress so far into graphene-based solar cells and this is going to continue well into the future. The ability to optimize various parameters makes graphene-based solar cells highly tuneable and adaptable to future challenges in solar research. Whether through improving existing solar cells, improving the properties of current non-graphene-based solar cells, or by creating a new range of graphene photovoltaics it is evident that graphene has a role in this exciting and rapidly advancing field.
References:
Huang X., Xiaoying Q., Boey F. and Zhang H., Graphene based composites, Chem Soc. Rev., 2012, 41, 666-686
Singh E., Nalwa H., Graphene-based bulk-heterojunction solar cells: A Review, Journal of Nanoscience and Nanotechnology, 2015, 15, 6237-6278
Li P., Chen C., Zhang J., Li S., Sun B., Bao Q., Graphene-based transparent electrodes for hybrid solar cells, Frontiers in Materials, 2014, 1, 26
Guo X., Lu G., Chen J., Graphene-based materials for photoanodes in dye-Sensitized solar cells, Frontiers in Energy Research, 2015, 3, 50
Ye Y., Dai L., Graphene-based schottky junction solar cells, J. Mater. Chem., 2012, 22, 24224
Feng T., Xie D., Lin Y., Zang Y., Ren T., Graphene based Schottky junction solar cells on patterned silicon-pillar-array substrate, App. Phys. Lett., 2011, 99, 233505
Jie W., Zheng F., Hao J., Graphene/gallium arsenide-based Schottky junction solar cells, App. Phys. Lett., 2013, 103, 233111
Miao X., Tongay S., Petterson M., Berke K., Rinzler A., Appleton B., Hebard A., High Efficiency Graphene Solar Cells by Chemical Doping, Nano Lett., 2012, 12(6), 2745-2750
Li X., Zhang S., Wang P., Zhong H., Wu Z., Chen H., Liu C., Lin S., High performance solar cells based on graphene-GaAs heterostructures, Nano Energy, 2015, 16, 310
Li X., Chen W., Zhang S., Wu Z., Wang P., Xu Z., Chen H., Yin W., Zhong H., Lin S., 18.5% efficient graphene/GaAs van der Waals heterostructure solar cell, Nano Energy, 2015, 16, 310-319
Yoshikawa K., Kawasaki H., Yoshida W., Irie T., Konishi K., Nakano K., Uto T., Adachi D., Kanematsu M., Uzu H., Yamamoto K., Silicon heterojunction solar cell with interdigitated back contacts for a photoconversion efficiency over 26%, 2017, Nature Energy, 2, 17032
- Forums
- ASX - By Stock
- GSL
- Ann: Greatcell Solar - Subscription Update
Ann: Greatcell Solar - Subscription Update, page-12
-
- There are more pages in this discussion • 13 more messages in this thread...
You’re viewing a single post only. To view the entire thread just sign in or Join Now (FREE)